Cerebral and spinal adhesive arachnoiditis
Arachnoiditis Overview
Arachnoiditis is an inflammatory condition affecting the arachnoid mater, one of the three meningeal layers surrounding the brain and spinal cord. This inflammation leads to thickening, scarring, and adhesions (abnormal connections) between the arachnoid and pia mater (leptomeninges), potentially involving nerve roots, blood vessels, and obstructing the flow of cerebrospinal fluid (CSF) [1]. Although termed "arachnoiditis," the inflammatory process typically involves adjacent meningeal layers and is not isolated solely to the arachnoid. Some consider it a form of chronic, localized serous meningitis due to the involvement of other layers and CSF pathways. However, its distinct clinical presentation, characterized by chronic pain, neurological deficits, and potential for CSF flow disturbances, justifies its classification as a specific condition.
Causes of Arachnoiditis
Arachnoiditis develops as a response to various insults that trigger inflammation within the subarachnoid space. Common causes and contributing factors include [2, 3]:
- Infections: Previous bacterial, fungal, viral, or parasitic meningitis or myelitis (inflammation of the spinal cord). Tuberculosis meningitis is a known cause.
- Spinal Surgery Complications: Particularly multiple or complex surgeries (failed back surgery syndrome), where bleeding into the subarachnoid space or direct irritation can occur. Dural tears during surgery are a risk factor.
- Spinal Anesthesia/Procedures: Epidural or spinal anesthesia (especially multiple attempts or traumatic taps leading to bleeding), myelography using older oil-based contrast agents like Pantopaque (now rare), or intrathecal injections (e.g., chemotherapy, steroids - especially depot preparations, although highly controversial).
- Trauma: Significant direct trauma to the spine or brain (TBI, spinal cord injury), leading to hemorrhage or inflammation in the subarachnoid space.
- Subarachnoid Hemorrhage: Blood breakdown products (e.g., iron) in the CSF are highly irritating and can induce an inflammatory response leading to adhesions.
- Chronic Intoxication/Chemical Irritation: Exposure to certain toxins or irritants introduced into the CSF space. Chronic alcoholism has been suggested as a contributing factor in some literature.
- Inflammatory Conditions: Systemic inflammatory diseases affecting the CNS (e.g., sarcoidosis). Reactive inflammation secondary to adjacent processes like chronic severe disc herniations or slow-growing tumors (e.g., schwannomas, meningiomas).
- Idiopathic: In a significant number of cases, a specific cause cannot be identified.
Morphologically, the arachnoid membrane becomes thickened, opaque, and fibrotic. Adhesive bands form between the arachnoid and pia mater, tethering nerve roots (in the spinal form) or encasing cranial nerves and blood vessels (in the cerebral form). These adhesions can obliterate the subarachnoid space, impede CSF flow, and lead to the formation of CSF-filled cysts (arachnoid cysts or loculations) [4].
Arachnoiditis is often described as diffuse, affecting a wide area, but frequently exhibits more severe, localized changes within that diffuse process, leading to focal symptoms. Impaired CSF circulation is a major consequence. Blockage of CSF outflow pathways (e.g., foramina of Luschka and Magendie in posterior fossa arachnoiditis) can cause obstructive hydrocephalus. Impaired absorption of CSF through inflamed or scarred arachnoid granulations can lead to communicating hydrocephalus.
Types of Arachnoiditis
Cerebral Arachnoiditis (Brain Membranes)
Cerebral arachnoiditis involves inflammation and scarring of the arachnoid mater surrounding the brain. It can be localized to specific areas like the cerebral convexity (surface), the basal cisterns (CSF spaces at the base of the brain), or the posterior cranial fossa. The clinical presentation results from a combination of generalized inflammatory effects, local compression or irritation of underlying brain tissue and cranial nerves by adhesions or cysts, and disturbances in CSF circulation leading to hydrocephalus or localized CSF trapping [5]. A common symptom across different locations is headache, often described as diffuse, persistent, and potentially worsening with maneuvers that increase intracranial pressure (hypertensive headache or 'shell' headache).
Convexital Cerebral Arachnoiditis
Convexital cerebral arachnoiditis affects the arachnoid mater overlying the surface (convexity) of the cerebral hemispheres, most commonly the anterior regions and areas around the central sulcus (rolandic area). Inflammation and adhesions here can directly irritate or compress the underlying cerebral cortex. This can lead to focal neurological deficits such as motor disturbances (monoparesis or hemiparesis due to involvement of the motor cortex) and sensory disturbances (paresthesias, numbness). Cortical irritation frequently manifests as focal seizures, which may sometimes generalize. Cyst formation within the adhesions can exacerbate compression and increase the likelihood of seizures, potentially progressing to status epilepticus in severe cases [6]. Diagnostic tools like Electroencephalography (EEG) can help localize epileptogenic foci, while neuroimaging (MRI) is essential to visualize the adhesions, CSF loculations, and any underlying cortical changes.
Optic Chiasmatic Arachnoiditis
Arachnoiditis localized to the basal cisterns is common, with the opto-chiasmatic region (around the optic nerves, optic chiasm, and base of the frontal lobes) being a frequent site. Opto-chiasmatic arachnoiditis is particularly significant due to its potential to cause severe and often irreversible vision loss. Common preceding causes include infections spreading from nearby sinuses (sinusitis), pharynx, or systemic infections like syphilis or tuberculosis, as well as traumatic brain injury or subarachnoid hemorrhage [5].
Pathologically, inflammation leads to the formation of dense adhesions and sometimes cysts that encase the optic nerves, chiasm, and adjacent structures like the pituitary stalk and hypothalamus. The process is rarely strictly localized, often extending subtly to surrounding basal cisterns. Damage to the optic pathways results from direct compression by adhesions/cysts, inflammatory involvement of the nerves/chiasm (optic neuritis component), and compromised blood supply (ischemia).
Clinically, opto-chiasmatic arachnoiditis typically presents with gradual, progressive visual loss, often starting unilaterally but usually becoming bilateral over weeks or months. This slow progression helps differentiate it from acute retrobulbar neuritis. The severity ranges from mild blurring to complete blindness. Pain behind the eyes (retrobulbar pain) may occur early in the course. Intracranial hypertension is usually mild or absent in this localized form.
Ophthalmological evaluation is key. Visual field testing (perimetry) reveals defects corresponding to the site of maximal involvement along the optic pathways. Common patterns include bitemporal hemianopia (indicating chiasmal compression), junctional scotomas, central scotomas (often bilateral), or concentric constriction of the visual fields [8].
Funduscopic examination is crucial. Optic nerve atrophy (pallor of the optic disc) develops over time and is seen in a majority of cases (60-65 percent), reflecting nerve damage. It can be primary (direct nerve damage) or secondary (following initial disc swelling). Papilledema (optic disc swelling due to raised ICP) is less common (10-13 percent) than atrophy in this predominantly basal form. Hypothalamic or pituitary dysfunction is typically absent unless the adhesions are extensive. Imaging of the sella turcica is usually normal, helping to differentiate from pituitary tumors.
Posterior Cranial Fossa Arachnoiditis
Arachnoiditis affecting the posterior cranial fossa is the most frequent form of localized cerebral arachnoiditis, often related to previous ear infections (otitis media, mastoiditis) or meningitis [5]. Its clinical presentation can closely mimic posterior fossa tumors (e.g., acoustic neuroma, meningioma, cerebellar tumor). Symptoms arise from involvement of the cerebellum, brainstem, and cranial nerves exiting in this region, as well as from obstruction of CSF flow leading to hydrocephalus.
Common locations include the cerebellopontine angle (CPA), cisterna magna, and surrounding the fourth ventricle outlets (foramina of Luschka and Magendie). CPA arachnoiditis typically involves cranial nerves VIII (hearing loss, tinnitus, vertigo), V (facial numbness/pain), and VII (facial weakness). Cerebellar signs like ataxia (unsteady gait, poor coordination), dysmetria, adiadochokinesia, and nystagmus are common. Brainstem compression can cause pyramidal signs or further cranial nerve palsies.
Obstruction of CSF outflow from the fourth ventricle by adhesions or cysts readily causes obstructive hydrocephalus, leading to symptoms of increased intracranial pressure (headache, nausea, vomiting, papilledema). The presentation depends on the balance between inflammation, adhesion/cyst formation, and hydrocephalus development. It can be acute, subacute, or chronic.
The acute form often presents predominantly with symptoms of increased ICP (severe headache, often occipital, vomiting, dizziness, papilledema, sometimes bradycardia), while focal neurological signs might be subtle or absent initially.
In the subacute or chronic forms, focal signs, particularly those related to CPA involvement, may predominate. Vestibular dysfunction is common, including spontaneous nystagmus (often unstable, may change direction, or be positional), gait instability with falling towards the affected side, and abnormal vestibular function tests (caloric or rotational testing may show disharmony). Hearing loss (sensorineural) is frequent with VIIIth nerve involvement. Palsies of cranial nerves V, VI, VII, IX, and X can occur. Pyramidal signs are usually mild unless there is significant brainstem compression.
Specific locations within the posterior fossa can yield distinct syndromes, such as isolated vestibular nerve involvement, prepontine arachnoiditis causing balance issues, or involvement of the jugular foramen affecting nerves IX, X, and XI.
Otogenic hydrocephalus secondary to posterior fossa arachnoiditis presents primarily with raised ICP symptoms, often accompanied by vestibular disturbances or mild cerebellar signs. CSF analysis might be normal or show low protein.
Rapidly progressive occlusive hydrocephalus can lead to acute crises with severe headache, vomiting, altered consciousness, and potentially papilledema and vision loss. A life-threatening complication is cerebellar tonsillar herniation through the foramen magnum due to high pressure, compressing the medulla and causing respiratory arrest.
Posterior fossa arachnoiditis can also be a challenging cause of intractable trigeminal neuralgia due to involvement of the Vth nerve root entry zone.
Spinal Arachnoiditis (Spinal Membranes)
Spinal arachnoiditis involves inflammation and scarring of the arachnoid mater surrounding the spinal cord and nerve roots within the spinal canal. Causes mirror those of cerebral arachnoiditis, with prior spinal surgery, spinal anesthesia/procedures, trauma, infection (meningitis, myelitis), and subarachnoid hemorrhage being common triggers [1]. It can also be associated with systemic inflammatory conditions.
Adhesions tether the spinal cord and nerve roots, impede CSF flow within the spinal subarachnoid space, and can compromise blood supply. This typically leads to chronic, progressive neurological symptoms. The clinical picture often resembles that of an extramedullary spinal cord tumor or spinal stenosis. Common features include:
- Chronic Pain: Often the most prominent and debilitating symptom. Typically neuropathic in character (burning, stinging, shooting pain), commonly affecting the back, legs, or perineum.
- Radiculopathy: Symptoms related to involvement of specific nerve roots, including radiating pain, numbness, tingling, and weakness in the distribution of the affected root(s).
- Myelopathy: Symptoms of spinal cord dysfunction due to compression, ischemia, or associated syrinx formation (fluid cavity within the cord). This includes weakness or spasticity in the limbs, sensory level deficits, gait disturbance, and bowel/bladder dysfunction.
Spinal arachnoiditis most frequently affects the lumbar and thoracic regions, often involving the cauda equina (nerve roots at the end of the spinal cord). The process commonly involves multiple nerve roots. Sensory disturbances can be patchy and variable. CSF analysis may show elevated protein (protein-cell dissociation) due to CSF stagnation below the level of obstruction, but pleocytosis is uncommon unless there is active inflammation. Myelography (CT or MR) is essential for diagnosis, classically showing clumping of nerve roots, adherence of roots to the thecal sac periphery ("empty sac" sign), loculated CSF collections, or blockage of contrast flow. MRI is the preferred imaging modality [9].
Spinal arachnoiditis is typically a chronic, often progressive condition that can be very difficult to treat effectively.
Diagnosis of Arachnoiditis
Diagnosing arachnoiditis requires integrating clinical findings (history, symptoms, neurological examination) with supportive evidence from diagnostic tests, primarily neuroimaging and sometimes CSF analysis. Establishing a definitive cause is often difficult.
Differential Diagnosis of Arachnoiditis
| Condition | Key Features / Distinguishing Points | Typical Imaging / Lab Findings |
|---|---|---|
| Arachnoiditis (Adhesive) | Chronic pain (often neuropathic), progressive neurological deficits (radiculopathy, myelopathy, cranial nerve palsies). History of potential trigger (infection, surgery, SAH, trauma, contrast). | MRI: Nerve root clumping/peripheral displacement ("empty thecal sac"), CSF loculations/cysts, obliterated subarachnoid space, +/- meningeal thickening/enhancement (often mild/absent in chronic stage). CSF: Often high protein (below block), normal/mild pleocytosis. |
| Meningitis (Bacterial, Fungal, TB) | Acute/subacute onset. Fever, headache, neck stiffness, altered mental status. May have cranial nerve palsies. | MRI: Diffuse leptomeningeal enhancement. CSF (LP crucial): Pleocytosis (neutrophils in bacterial, lymphocytes in viral/TB/fungal), low glucose (bacterial, TB, fungal), high protein. Positive Gram stain/culture/PCR. |
| Leptomeningeal Carcinomatosis / Metastases | History of known primary cancer. Multifocal neurological signs (cranial nerves, spinal roots, hydrocephalus). Headache, cognitive changes. Subacute onset. | MRI: Nodular or linear leptomeningeal enhancement, may involve nerve roots/cauda equina ("sugar coating"). CSF: Malignant cells on cytology (may need repeat LPs), high protein, low glucose. Systemic staging identifies primary. |
| Spinal Stenosis (Degenerative) | Neurogenic claudication (leg pain/weakness with walking, relieved by rest/flexion). Back pain common. Older age group typically. No history of inflammatory trigger usually. | MRI/CT: Narrowing of spinal canal/foramina due to disc bulge, facet hypertrophy, ligamentum flavum thickening compressing thecal sac/roots. No primary meningeal changes or diffuse root clumping. |
| Spinal Cord Tumor (Intradural-Extramedullary - Meningioma, Schwannoma) | Progressive myelopathy/radiculopathy depending on location. Pain common. Usually focal deficit pattern. (Related info: Spinal Cord Diseases) | MRI: Well-defined, often intensely contrast-enhancing *mass* displacing spinal cord/nerve roots. Meningioma often dural-based; schwannoma arises from root ("dumbbell" shape possible). No diffuse adhesions/root clumping typically. |
| Spinal Cord Tumor (Intramedullary - Ependymoma, Astrocytoma) | Progressive myelopathy (sensory level, weakness, bowel/bladder changes). Pain less typical initially than extramedullary. Usually central cord symptoms. (Related info: Spinal Cord Diseases) | MRI: Expansion of spinal cord, intrinsic T2 hyperintensity, variable contrast enhancement pattern (ependymoma often enhances intensely). May have associated syrinx or tumor cysts. |
| Syringomyelia | Fluid-filled cavity within spinal cord. Often causes central cord syndrome (dissociated sensory loss - pain/temp affected first, cape-like distribution), weakness/atrophy. Can be secondary to arachnoiditis. | MRI: Clearly defines intramedullary cystic cavity (syrinx). Important to search for underlying cause (Chiari malformation, tumor, post-traumatic, post-inflammatory/arachnoiditis). |
| Transverse Myelitis | Acute/subacute onset of myelopathy (weakness, sensory level, bowel/bladder dysfunction). Often post-infectious or autoimmune (e.g., MS, NMO, MOGAD). Pain can occur. | MRI: Intramedullary T2 hyperintensity +/- contrast enhancement, typically spanning several segments centrally. CSF may show pleocytosis/high protein/oligoclonal bands. Specific antibodies (AQP4, MOG) for NMO/MOGAD. |
| Intracranial Hypotension (often due to Spontaneous CSF Leak) | Orthostatic headache (worse upright, better lying down) is hallmark. Caused by CSF leak (spontaneous or post-LP/surgery). Can cause diffuse pachymeningeal (dural) enhancement, *not* leptomeningeal. | MRI: Diffuse, smooth pachymeningeal enhancement, sagging brainstem, subdural collections/hygromas. LP (if done) shows low opening pressure. Myelography/CT/MRI myelography may identify leak site. |
| Guillain-Barré Syndrome (GBS) / CIDP | Acute (GBS) or chronic (CIDP) polyradiculoneuropathy. Ascending weakness, areflexia, sensory changes. Primarily affects peripheral nerve roots, not meninges directly. Pain common. | MRI: May show enhancement of spinal nerve roots/cauda equina (esp. anterior roots). CSF: Albuminocytologic dissociation (high protein, normal/low cells) - classic finding. Nerve conduction studies diagnostic. |
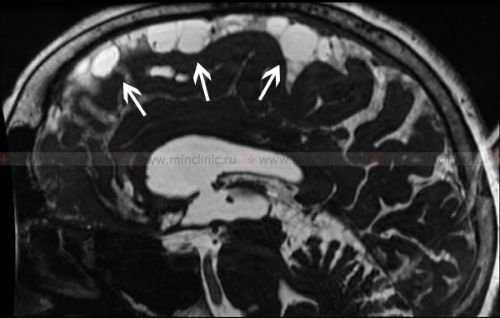
Magnetic resonance imaging (MRI) is the primary tool for visualizing the changes associated with arachnoiditis, such as meningeal thickening, adhesions, nerve root clumping, or CSF loculations. This video may demonstrate typical MRI findings.
Key diagnostic approaches include:
- Clinical Evaluation: A detailed history focusing on potential causes (infection, surgery, trauma, hemorrhage, anesthesia) and the nature/progression of symptoms (chronic pain, specific neurological deficits) is crucial. The neurological examination aims to localize the deficits (cranial nerves, motor/sensory pathways, cerebellar function, spinal nerve roots).
- Neuroimaging:
- MRI of the Brain or MRI of the Spinal Cord (with and without Gadolinium contrast): The modality of choice [10, 9]. Findings may include:
- Thickening and enhancement of the leptomeninges (arachnoid/pia) after gadolinium administration (enhancement may be present in active inflammation but often absent or mild in chronic, purely adhesive stages).
- Obliteration of normal CSF spaces or abnormal accumulation/loculation of CSF.
- *Spinal specific:* Clumping of nerve roots centrally within the thecal sac, adherence of roots peripherally against the thecal sac ("empty sac" sign), or nerve roots encased in thickened meninges.
- Formation of arachnoid cysts or CSF loculations (appearing as fluid signal intensity collections on T2-weighted images, may have thin enhancing walls).
- Associated findings like hydrocephalus (cerebral), syringomyelia (spinal cord cavity), or changes in the underlying brain/spinal cord parenchyma (e.g., edema, T2 signal change suggesting gliosis or myelomalacia).
- CT Myelography: May be used for spinal arachnoiditis if MRI is contraindicated or inconclusive. Can show blockage of contrast flow, irregular margins of the contrast column, nerve root clumping, root sleeve amputation, or contrast pooling within cysts/loculations. Less sensitive than MRI for visualizing meningeal thickening or intrinsic cord changes.
- Plain X-rays or CT of the Skull/Spine: Generally not useful for diagnosing arachnoiditis itself but may show signs of increased ICP (e.g., sella erosion in chronic hydrocephalus) or bony changes related to prior surgery or trauma.
- MRI of the Brain or MRI of the Spinal Cord (with and without Gadolinium contrast): The modality of choice [10, 9]. Findings may include:
- Cerebrospinal Fluid (CSF) Analysis: Obtained via lumbar puncture (LP), only if considered safe (i.e., no signs of high ICP or complete spinal block confirmed on imaging). Findings are often nonspecific in chronic adhesive arachnoiditis. May show elevated protein (especially below a spinal block due to CSF stagnation), mild pleocytosis (increased cells, usually lymphocytes) in some active inflammatory phases, or be entirely normal. Primarily useful to rule out active infection (meningitis) or malignancy (leptomeningeal carcinomatosis).
- Electroencephalography (EEG): May help localize epileptogenic activity in patients with convexital cerebral arachnoiditis presenting with seizures. Can show diffuse slowing in cases with significant hydrocephalus or diffuse cerebral involvement but is generally non-specific for arachnoiditis itself.
- Ophthalmological Examination: Including fundus examination (for papilledema or optic atrophy) and visual field testing (perimetry), is essential for suspected optic-chiasmatic or posterior fossa arachnoiditis causing visual symptoms or signs of raised ICP.
Differentiating arachnoiditis from other conditions, particularly tumors (meningioma, schwannoma, ependymoma, metastatic disease), can be challenging based on imaging alone, especially for localized cystic forms. Clinical history, CSF analysis (especially cytology for malignancy), and sometimes surgical biopsy are needed. Compared to tumors, arachnoiditis often presents with more diffuse or multi-level involvement, less intense contrast enhancement in chronic stages (though active inflammation can enhance), and a history suggestive of an inflammatory trigger.
Treatment of Arachnoiditis
The treatment of arachnoiditis is often challenging and primarily focuses on managing symptoms, particularly chronic pain and neurological deficits, as reversing the underlying scarring and adhesions is difficult or impossible. There is no definitive cure [11].
Treatment strategies include:
- Medical Management:
- Pain Control: This is often the mainstay and requires a multimodal approach. Options include neuropathic pain medications (e.g., gabapentin, pregabalin, tricyclic antidepressants like amitriptyline, SNRIs like duloxetine), NSAIDs for inflammatory components, and sometimes, cautiously, opioids for severe, refractory pain (long-term use is problematic due to tolerance, dependence, and side effects).
- Anti-inflammatory Agents: Corticosteroids (oral or IV) may be used short-term during documented acute inflammatory flares or potentially postoperatively, but their long-term benefit in chronic adhesive arachnoiditis is unproven and controversial due to significant side effects. Other immunomodulators are rarely used unless an underlying systemic inflammatory condition is present.
- Muscle Relaxants: For associated muscle spasms (e.g., baclofen).
- Physical Therapy: Gentle exercise (avoiding exacerbation), stretching, hydrotherapy, and TENS (transcutaneous electrical nerve stimulation) may help manage pain, maintain function and mobility, and prevent deconditioning.
- Interventional Pain Management:
- Epidural steroid injections (use is highly controversial and potentially risky in established arachnoiditis, may worsen condition for some).
- Spinal cord stimulation (SCS): Implantation of a device to deliver electrical impulses to the dorsal columns of the spinal cord may provide significant pain relief for some patients with chronic neuropathic pain predominantly in the limbs [12].
- Intrathecal drug delivery systems (Pain Pumps): Implanted pumps delivering pain medication (e.g., morphine, ziconotide, baclofen) directly into the CSF space may be considered for severe, refractory pain when other options fail, but require careful patient selection and management [12].
- Adhesiolysis (Racz procedure): Percutaneous lysis of epidural adhesions with hypertonic saline/local anesthetic/steroid injection; efficacy specifically for intradural arachnoiditis is questionable and carries risks.
- Surgical Intervention: Surgery is generally reserved for specific complications or progressive neurological deficits and aims to decompress neural structures or restore CSF flow, rather than "cure" the arachnoiditis itself. Procedures are often technically challenging with variable outcomes and risk of worsening symptoms or adhesion reformation [2, 6]. Options include:
- Lysis of Adhesions: Microsurgical dissection to free up tethered nerve roots or spinal cord. Results are often temporary as adhesions tend to reform. Carries significant risks including nerve injury and CSF leak. Rarely performed now for diffuse disease.
- Cyst Fenestration or Excision: Creating openings in or removing symptomatic arachnoid cysts/loculations causing significant compression.
- Dural Grafting / Expansile Duraplasty: Occasionally used after adhesion lysis or cyst removal to try and expand the space and potentially prevent recurrence, with limited proven success.
- CSF Shunting (e.g., Ventriculoperitoneal [VP] shunt): Necessary for treating associated symptomatic hydrocephalus.
- Syringosubarachnoid or Syringoperitoneal Shunt: For draining a symptomatic associated syrinx causing progressive myelopathy.
- Surgery for Optic-Chiasmatic Arachnoiditis: Microsurgical decompression of the optic nerves and chiasm by lysing adhesions and fenestrating cysts may be attempted urgently if vision is rapidly declining, but outcomes regarding vision recovery are variable and often disappointing; risks include further damage [5].
- Management of Underlying Cause: If an ongoing infectious or specific inflammatory process is identified (rare in chronic stage), targeted treatment (e.g., antibiotics, specific immunomodulation) is necessary.
Pneumoencephalography, mentioned historically in older literature for diagnosis and sometimes attempted therapy (air insufflation), is obsolete and no longer used, having been replaced by MRI and CT.
The prognosis for arachnoiditis is highly variable but often guarded, particularly for the spinal form. While generally not directly life-threatening (unless complicated by severe untreated hydrocephalus or brainstem compression), it frequently causes chronic, debilitating pain and progressive neurological impairment that significantly impacts quality of life. Functional recovery after treatment is often limited, especially in chronic, extensive cases with established neurological deficits. Early diagnosis and aggressive management of potential causes (e.g., prompt treatment of meningitis, careful surgical technique, avoiding older myelographic agents) may help prevent its development.
![]() Attention! Arachnoiditis is a complex condition often leading to chronic pain and disability. Diagnosis requires careful clinical evaluation and specialized imaging. Treatment is challenging and requires a multidisciplinary approach involving neurologists, neurosurgeons, pain management specialists, and physical therapists. Self-diagnosis or reliance on unproven therapies is strongly discouraged. Consult experienced medical professionals for accurate diagnosis and management options.
Attention! Arachnoiditis is a complex condition often leading to chronic pain and disability. Diagnosis requires careful clinical evaluation and specialized imaging. Treatment is challenging and requires a multidisciplinary approach involving neurologists, neurosurgeons, pain management specialists, and physical therapists. Self-diagnosis or reliance on unproven therapies is strongly discouraged. Consult experienced medical professionals for accurate diagnosis and management options.
References
- Wright MH, Denney LC. A comprehensive review of spinal arachnoiditis. Orthop Nurs. 2003 May-Jun;22(3):215-9; quiz 220-1. doi: 10.1097/00006416-200305000-00008
- Aldrete JA. Chronic Adhesive Arachnoiditis. Springer; 2011.
- Doležal M, Vizváry Z, Srp A, Chaloupský R. Spinal adhesive arachnoiditis. Review. Neuro Endocrinol Lett. 2019;40(2):61-66.
- Pantanowitz L, Hatae M, Fitt G, Miller DC. Pathology of arachnoiditis. Surg Neurol Int. 2011;2:90. doi: 10.4103/2152-7806.82567
- Chapter 32: Inflammatory diseases. In: Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019.
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2020.
- Osborn AG, Salzman KL, Jhaveri MD, et al. Osborn's Brain. 2nd ed. Elsevier; 2018.
- Walsh FB, Hoyt WF. Clinical Neuro-Ophthalmology. 4th ed. Williams & Wilkins; 1982-1991.
- Anderson TL, et al. Imaging of Spinal Arachnoiditis. Neuroimaging Clin N Am. 2017 Feb;27(1):93-106. doi: 10.1016/j.nic.2016.09.005
- Jinkins JR. MR of adhesive arachnoiditis. AJNR Am J Neuroradiol. 1993 May-Jun;14(3):764-6.
- Guyer DW, et al. Arachnoiditis. Orthop Clin North Am. 2019 Apr;50(2):181-191. doi: 10.1016/j.ocl.2018.12.003
- Deer TR, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for Intrathecal Drug Delivery: Guidance for Improving Safety and Reducing Serious Adverse Events. Neuromodulation. 2017;20(2):155-176. doi: 10.1111/ner.12579
See also
- Anatomy of the nervous system
- Central nervous system infection:
- Brain abscess (lobar, cerebellar)
- Eosinophilic granuloma, Langerhans cell histiocytosis (LCH), Hennebert's symptom
- Epidural brain abscess
- Sinusitis-associated intracranial complications
- Otogenic intracranial complications
- Sinusitis-associated ophthalmic complications
- Bacterial otogenic meningitis
- Subdural brain abscess
- Sigmoid sinus suppurative thrombophlebitis
- Cerebral 3rd Ventricle Colloid Cyst
- Cerebral and spinal adhesive arachnoiditis
- Corticobasal Ganglionic Degeneration (Limited Brain Atrophy)
- Encephalopathy
- Headache, migraine
- Traumatic brain injury (concussion, contusion, brain hemorrhage, axonal shearing lesions)
- Increased intracranial pressure and hydrocephalus
- Parkinson's disease
- Pituitary microadenoma, macroadenoma and nonfunctioning adenomas (NFPAs), hyperprolactinemia syndrome
- Spontaneous cranial cerebrospinal fluid leak (CSF liquorrhea)